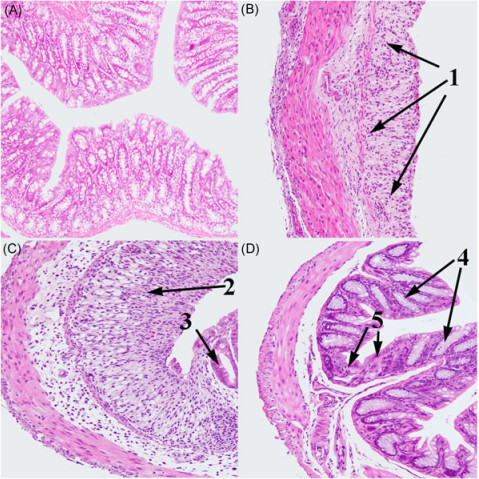
[4–6] However, over-activation of inflammasomes will result in an inflammatory disorder of the intestinal tract.[2,7]
Zearalenone (ZEA) is a nonsteroidal estrogenic mycotoxin and is mainly produced by several Fusarium species. It can widely contaminate cereal crops, including corn, barley, wheat, oats, sorghum, and sesame grains.
[8] It has been reported that ZEA is associated with many mycotoxicoses in farm animals.[9] ZEA accounts annually for millions of dollars in pig breeding losses worldwide.[10] If an animal ingests ZEA‐ contaminated feed, the intestine will be exposed to high concentrations of ZEA. The adverse influences of ZEA on the gut have been confirmed.[11–13] In our previous study, we proved that ZEA exposure could induce mitochondrial damage by reducing antioxidant enzyme activities, accumulation of reactive oxygen species (ROS), and decreasing mitochondrial membrane potential (MMP).
[14] The mitochondria‐derived components could trigger NLRP3 inflammasome activation to convert the proinflammatory cytokines IL‐1β and IL‐18 into their active forms, which were the central players in the pathogenesis of colitis and mediated ZEA‐induced intestinal inflammation in mouse colon, which were characterized by significant inflammatory cell infiltration and tissue damage.
[7] Because ZEA can induce intestinal inflammation, we specu-lated that the ingestion of large amounts of ZEA‐contaminated feed might aggravate inflammatory reactions in the gastrointestinal tract. To verify this notion, we investigated the influence of ZEA on the dextran sulfate sodium (DSS)‐induced colitis model both in vitro and in vivo. DSS has been widely used to induce colitis in model mice.
[15] And this model is particularly useful for research into the role of innate immune mechanisms in intestinal inflammation.[16] Surprisingly, the results indicated that, instead of aggravating DSS‐induced colitis, ZEA relieved the inflammatory reaction in colon tissue.
REFEREN CES
[1]F. Martinon, A. Mayor, J. Tschopp, Annu. Rev. Immunol. 2009, 27, 229.
[2]N. Zmora, M. Levy, M. Pevsner‐Fishcer, E. Elinav, Mucosal Immunol. 2017, 10, 865.
[3]A. C. Villani, M. Lemire, G. Fortin, E. Louis, M. S. Silverberg, C. Collette,
N.Baba, C. Libioulle, J. Belaiche, A. Bitton, D. Gaudet, A. Cohen, D. Langelier, P. R. Fortin, J. E. Wither, M. Sarfati, P. Rutgeerts, J. D. Rioux, S. Vermeire, T. J. Hudson, D. Franchimont, Nat. Genet. 2009, 41, 71.
[4]M. Lamkanfi, V. M. Dixit, Cell 2014, 15(5), 1013.
[5]A. Lu, V. G. Magupalli, J. Ruan, Q. Yin, M. K. Atianand, M. R. Vos, G. F. Schr?der, K. A. Fitzgerald, H. Wu, E. H. Egelman, Cell 2014, 156 (6), 1193.
[6]Y. Ishiguro, J. Gastroenterol 1999, 34(6), 66.
[7]W. Fan, Y. Lv, S. Ren, M. Shao, T. Shen, K. Huang, J. Zhou, L. Yan, S. Song, Chemosphere 2018, 190, 272.
[8]C. Tabuc, D. Marin, P. Guerre, T. Sesan, J. D. Bailly, J. Food Prot 2009, 72(3), 662.
[9]J. Finkgremmels, H. Malekinejad, Anim. Feed Sci. Tech 2007, 137 (3), 326.
[10]H. Jo, C. Kong, M. Song, B. G. Kim, Anim. Feed Sci. Tech 2016, 219, 77.
[11]M. Liu, R. Gao, Q. Meng, Y. Zhang, C. Bi, A. Shan, Plos One 2014, 9(9), e106412.
[12]P. Pinton, I. Oswald, Toxins 2014, 6(5), 1615.
[13]H. Robert, D. Payros, P. Pinton, V. Théodorou, M. Mercier‐Bonin, I. P. Oswald, J. Toxicol. Environ. Health 2017, 20(5), 249.
[14]W. Fan, T. Shen, Q. Ding, Y. Lv, L. Li, K. Huang, L. Yan, S. Song, J. Biochem. Mol. Toxicol 2017, 31, e21944.
[15]I. Okayasu, S. Hatakeyama, M. Yamada, T. Ohkusa, Y. Inagaki, R. Nakaya, Gastroenterology. 1990, 98(3), 694.
[16]C. Bauer, P. Duewell, C. Mayer, H. A. Lehr, K. A. Fitzgerald, M. Dauer, J. Tschopp, S. Endres, E. Latz, M. Schnurr, Gut. 2010, 59(9), 1192.
[17]I. C. Allen, J. E. Wilson, M. Schneider, J. D. Lich, R. A. Roberts, J. C. Arthur, R. M. T. Woodford, B. K. Davis, J. M. Uronis, H. H. Herfarth,
C.Jobin, A. B. Rogers, J. P. Y. Ting, Immunity. 2012, 36(5), 742.
[18]S. Mariathasan, K. Newton, D. M. Monack, D. Vucic, D. M. French, W.
P.Lee, M. Roosegirma, S. Erickson, V. M. Dixit, Nature. 2004, 430 (6996), 213.
[19]A. M. Nour, Y. L. Yeung, Infect. Immun 2009, 77(3), 1262.
[20]S. Brase,? A. Encinas, J. Keck, C. F. Nising, Chem. Rev. 2009, 109 (9), 3903.
[21]C. M. Brown, T. A. Mulcahey, N. C. Filipek, P. M. Wise, Endocrinology 2010, 151(10), 4916.
[22]D. R. V. Jp, H. H. Patel, M. A. Perez‐Pinzon, H. M. Bramlett, A. P. Raval, J. Neurochem 2015, 136(3), 492.
[23]W. Huan, Z. Tianzhu, L. Yu, W. Shumin, Inflammation. 2017, 40(3), 1.